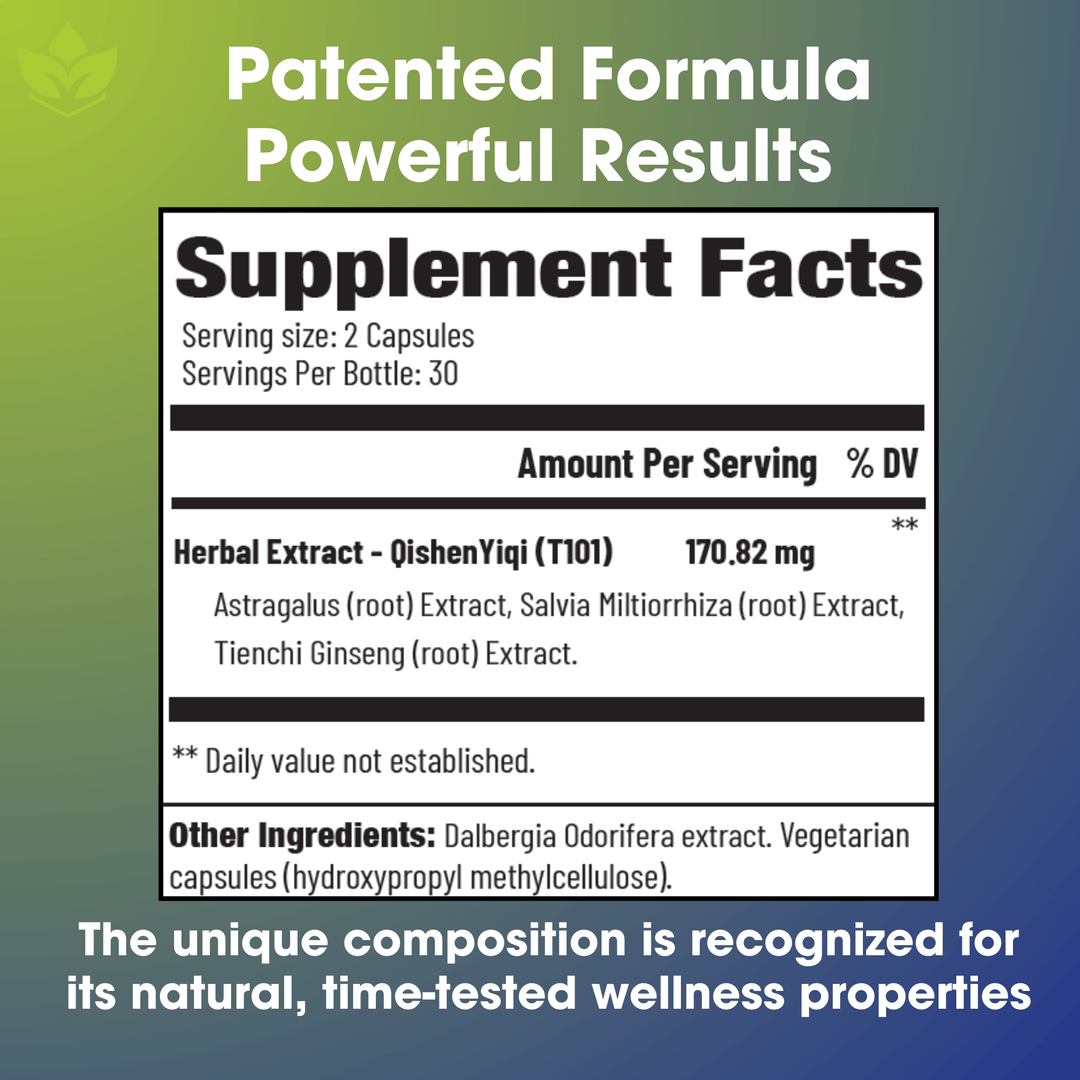
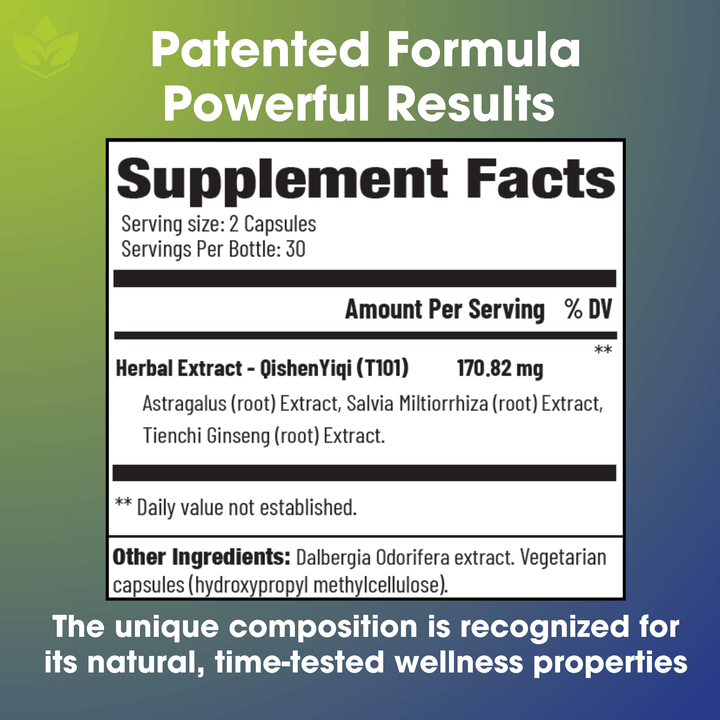
Ingredients:
• Herbal Extract- QishenYiqi (T101): Astragalus (root) Extract, Salvia Miltiorrhiza (root) Extract, Tienchi Ginseng (root) Extract.
• Other Ingredients: Dalbergia Odorifera extract. Vegetarian capsules (hydroxypropyl methylcellulose).
• DOES NOT CONTAIN: Wheat, nuts, gluten, sugar, soy, corn, yeast, milk, shellfish.
Direction:
• Take two (2) capsules daily with a meal.
• For best results, recommend taking daily for at least three (3) months.
Caution:
• For adults only.
• Consult your healthcare professional if you have any medical conditions or use any medications.
• Stop use if you have allergic reactions towards NatureKue CardioSupport or its ingredients. Do not use if you are pregnant, attempting to become pregnant, or if nursing.
• READ the entire label before use. KEEP THIS OUT OF REACH OF CHILDREN. • Do not use it if the safety seal is damaged or missing. Store in a dry place at room temperature.* These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, cure or prevent any disease.